How to Find Work Function Given Wavelength
Wavelength Speed of the waveFrequency of the wave As mentioned above all the quantities are. The wavelength to frequency formula is given by.

The Work Function Of Cs Is 2 14ev Find A Threshold Frequency For Cs B Wavelength Of Incident Youtube
H c λ h c λ ω 0 2 ω 0.
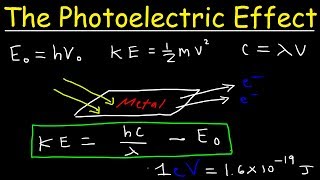
. Two of the easiest points to find are the crest the high point of a wave or. How to Find the Work Function of a Material Given Experimental Data from the Photoelectric Effect Step 1. We have Φ h ν o hcλ o.
F f f 075 wavess So the frequency of the wave is 075 waves. This chemistry video tutorial explains how the photoelectric effect works. The wave travels a distance of 3m in 4s.
λ λ 2. Remember that the work function of a metal is the energy required to make an electron free from the surface. λ 03 m.
Recall that the formula for work function given incident photon wavelength is 𝑊 ℎ 𝑐 𝜆 𝐸. When you substitute this into the equation Ek hv - work function you will get the equation hv work. A small plate of a metal work function 117 eV is placed at a distance of 2 m from a monochromatic light source of wavelength 48 x 10-7 m and power 10 watt.
It also explains how to use the work function of metals to calculate the threshol. When radiation of certain wavelength shines on the cathode of the photoelectric cell the photocurrent produced can be reduced to zero by applying stopping potential of 263. In one experiment the electron speed was twice the.
To find the length of a wave one has to measure from the same points on two consecutive waves. Hcwavelength workfunction KE of Energy of electron Now workfunction is given convert it to J Then the velocity of electron is given so find out the KE of electron using. Its typical to need to perform a unit conversion on the wavelength value in order to get it to work in the equation.
The wave speed is calculated by v 3 4. A video solution for a problem in Modern Physics class 12th - Finding stopping potential for a given wavelength using einsteins photoelectric equationJEE M. Say we know the velocity of the electron once ejected.
So the correct answer is option C. The wavelength of the wave is 20 m Furthermore we have to rearrange the formula for calculating the answer. Speed Frequency x Wavelength.
H Φλ o c 63 x 16 x 10-19 x 1972 x 10-10 3 x 10 8 6625 x 10-34 Js. Just plug in the waves speed and frequency to solve for the wavelength. Use the data to find the equation of the line in slope - intercept form.
This is the value of threshold wavelength when the work function of the metal 2 ω 0. Work functionhf cf The Attempt at a Solution Given the wavelength I solved for f 3x10 8 262x10 -9 115x10 15 Then times by Plancks constant to get work function. To calculate wavelength use the formula wavelength speed divided by frequency.
First convert nm to m. So the work function gives the minimum energy required from a photon to knock an electron out of the surface of a metal. Nano- is 10 -9 so all you need to do is.
Because you are looking for the longest wavelength set Ek 0. The wavelength formula is given by λ v f. The value of Plancks constant is 6625 x 10-34 Js.
By dividing equation 2 by 3 then we get. A metal received light with wavelength 300 n m in experiment A and light with wavelength 500 n m in experiment B. To find the work function of the metal we can substitute the graphs horizontal intercept value into.

Photoelectric Effect Work Function Threshold Frequency Wavelength Speed Kinetic Energy Electr Youtube

Pin By Emonerd On Physics Quantum Physics Physics 101 Quantum Physics Physics

Comments
Post a Comment